Beer Lambert Law Calculator
Use Our Beer-Lambert law calculator to calculate the absorbance of light as it passes through any substance.
You can also use this calculator to determine the concentration of a solution using Beer's law.
To Calculate :
Absorbance :
Path Length :
cm
Concentration :
M
Coefficient :
M-1cm-1
Result
x
If you use this great tool then please comment and/or like this page.
Average Rating: Tool Views: 231
Average Rating: Tool Views: 231
Subscribe for Latest Tools
How to use this Beer Lambert Law Calculator Tool?
How to use Yttags's Beer Lambert Law Calculator?
- Step 1: Select the Tool
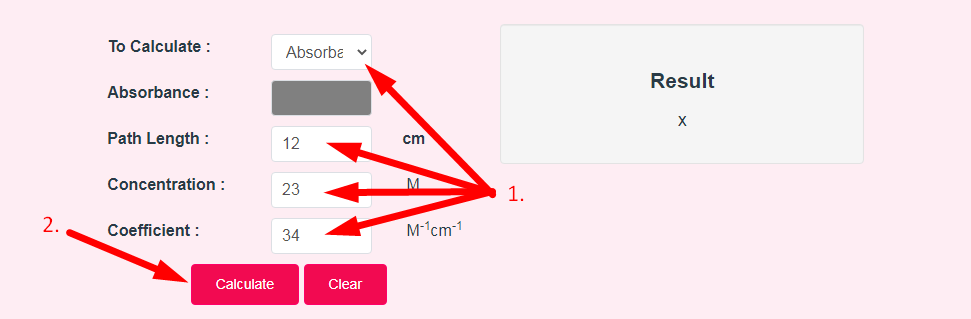
- Step 2: Enter The Following Options And Click On Calculate Button
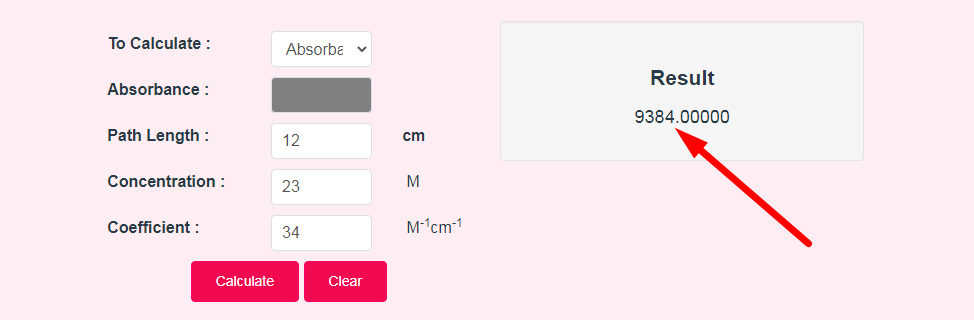
- Step 3: Check Your Beer Lambert Law Calculator Result
If you want to link to Beer Lambert Law Calculator page, please use the codes provided below!
FAQs for Beer Lambert Law Calculator
What is a Beer Lambert Law Calculator?
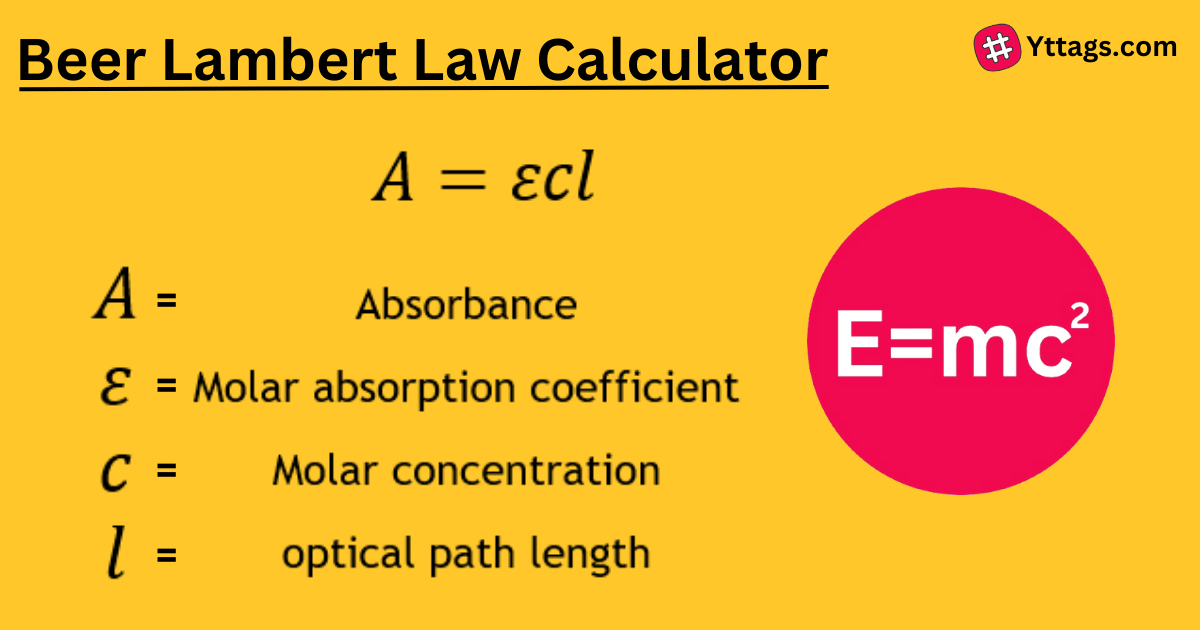
A Beer-Lambert Law calculator is a tool used to determine the concentration of a substance in a solution based on the absorbance of light at a specific wavelength, according to the Beer-Lambert Law.
What are limitations to Beer-Lambert law?
Causes of nonlinearity include: deviations in absorptivity coefficients at high concentrations (>0.01M) due to electrostatic interactions between molecules in close proximity. scattering of light due to particulates in the sample. fluoresecence or phosphorescence of the sample.
What is the Beer-Lambert law used to calculate?
The Beer-Lambert law states that there is a linear relationship between the concentration and the absorbance of the solution, which enables the concentration of a solution to be calculated by measuring its absorbance.
What is the purpose of the Beer-Lambert law practical?
Beer-Lambert Law derivation helps us define the relationship between the intensity of visible UV radiation and the exact quantity of substance present.
What are the causes of deviation from Beer's law?
These deviations are due to: (1) chemical reasons arising when the absorbing compound, dissociates, associates, or reacts with a solvent to produce a product having a different absorption spectrum, (2) the presence of stray radiation, and (3) the polychromatic radiation.